OCR Specification focus:
‘Analyse enzyme and substrate concentration effects; design serial dilutions and rate investigations.’
Understanding how enzyme and substrate concentrations influence reaction rates is crucial for analysing enzyme kinetics and mastering experimental techniques used to investigate these relationships in biological systems.
Enzyme and Substrate Concentration Effects
Enzyme Concentration and Reaction Rate
Increasing enzyme concentration raises the number of active sites available for substrate binding. This enhances the rate of reaction, provided substrate molecules are abundant. However, beyond a certain point, the reaction rate plateaus because substrate concentration becomes the limiting factor.
Key concept: At constant substrate concentration, the rate of reaction is directly proportional to enzyme concentration until the substrate is depleted.
When enzyme concentration is low, each enzyme molecule works continuously as substrates are abundant.
As enzyme concentration increases, the likelihood of enzyme–substrate complex formation also rises.
Once all substrates are bound and converted rapidly, the system reaches maximum rate (Vmax) for that substrate concentration.
Substrate Concentration and Reaction Rate
At a fixed enzyme concentration, increasing substrate concentration initially increases the rate of reaction due to more frequent enzyme–substrate collisions. This continues until all active sites are occupied.
Saturation Point: The substrate concentration at which all enzyme active sites are occupied, and the reaction rate can no longer increase.
At saturation, further substrate addition has no effect because the enzymes are already operating at maximum turnover rate. The graph of rate against substrate concentration shows a hyperbolic curve that flattens at Vmax.
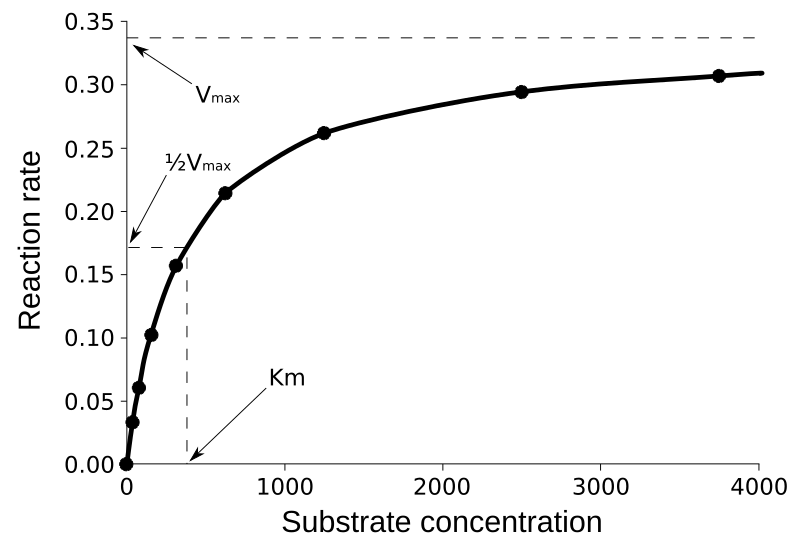
Michaelis–Menten saturation curve showing reaction rate versus substrate concentration at constant enzyme concentration. Rate rises rapidly at low [S] and approaches Vmax as active sites become saturated. Axes and key features are clearly indicated for quick interpretation. Source.
Rate of Reaction and Limiting Factors
The rate of enzyme-catalysed reactions depends on several limiting factors: enzyme concentration, substrate concentration, temperature, and pH. When investigating concentration effects, all other variables must remain constant to ensure a valid test.
At low substrate concentrations, substrate availability limits the rate.
At high substrate concentrations, enzyme active sites are the limiting factor.
At low enzyme concentrations, few active sites are available to catalyse reactions efficiently.
To achieve reliable and reproducible data, experiments must be conducted under controlled conditions, ensuring consistent temperature, pH, and enzyme stability.
Measuring Enzyme Activity
Observable Changes
To determine the rate of enzyme activity, measurable indicators of product formation or substrate loss are required.
Common measurable changes include:
Change in colour (e.g. starch to maltose using iodine test).
Gas volume (e.g. catalase breaking down hydrogen peroxide).
Mass change (e.g. breakdown of substrates producing gaseous products).
Turbidity (e.g. casein breakdown in milk becoming clearer).
The rate is typically expressed as change in measurable quantity per unit time.
EQUATION
—-----------------------------------------------------------------
Rate of Reaction (R) = Change in Quantity / Time Taken
R = Reaction rate (units vary: cm³ s⁻¹, mol dm⁻³ s⁻¹, etc.)
Quantity = Measured product or substrate change
Time = Duration of observation (seconds or minutes)
—-----------------------------------------------------------------
Designing Serial Dilutions
Purpose of Serial Dilutions
Serial dilutions are used to create a range of substrate or enzyme concentrations systematically, enabling investigation of how concentration affects reaction rate. Each dilution decreases the concentration by a consistent factor, often ×0.5 or 1/10, ensuring precise gradient generation.
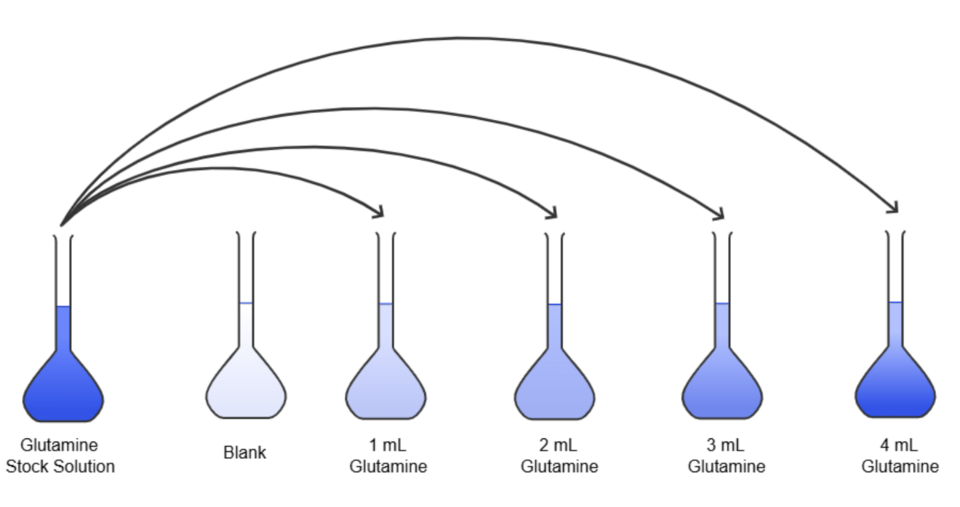
Stepwise serial dilution from a concentrated stock to lower, known concentrations. Each tube represents a fixed dilution factor, supporting precise preparation of enzyme or substrate ranges. Labels are clear and limited to essentials for practical work. Source.
Steps in serial dilution:
Label a series of test tubes (e.g. 1–5).
Add a fixed volume of solvent (usually distilled water) to each.
Add the enzyme or substrate solution to the first tube and mix thoroughly.
Transfer a measured volume from the first tube to the second, mix, and repeat sequentially.
Each tube now contains a predictable concentration reduction.
Serial Dilution: A stepwise dilution method that reduces the concentration of a solution by a consistent ratio between each successive sample.
This method provides a quantitative relationship between concentration and reaction rate, essential for drawing accurate conclusions.
Core Practical Skills
Variables and Controls
When investigating enzyme and substrate concentration effects, maintaining experimental validity requires careful control of variables:
Independent variable: enzyme or substrate concentration.
Dependent variable: rate of reaction.
Control variables: temperature, pH, volume, and mixing time.
Include a negative control (e.g. denatured enzyme or no enzyme) to confirm that observed changes are due to enzyme activity.
Reproducibility and Reliability
To ensure reliable results:
Repeat each test at least three times and calculate mean rates.
Identify and exclude anomalous results.
Plot data with rate vs. concentration to visualise trends.
Calculate initial rates from the steepest part of the graph to compare enzyme efficiency.
Data Presentation
When presenting enzyme kinetics data:
Use a line graph for continuous data (rate vs. concentration).
Label axes clearly with units (e.g. mol dm⁻³ for concentration).
Indicate Vmax and note where the curve levels off to show saturation.
If both enzyme and substrate concentrations are altered, separate graphs should be produced to identify which factor limits the rate.
Experimental Techniques in Rate Investigations
Practical Considerations
Reliable enzyme investigations require accurate measurement and consistency. Essential techniques include:
Using micropipettes for precise liquid handling.
Maintaining a constant temperature using a water bath.
Timing reactions accurately with a stopwatch.
Measuring product or colour change with colorimetry for quantitative analysis.
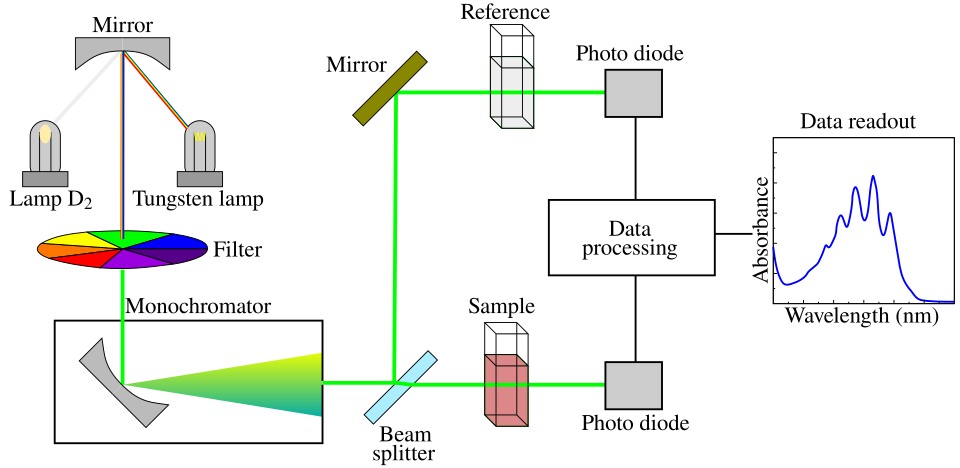
Schematic of a UV/Vis spectrophotometer used for colorimetric assays: a selected wavelength passes through a cuvette, and the detector measures transmitted light to calculate absorbance. This underpins quantitative rate measurements in enzyme practicals. The diagram is intentionally minimal to match syllabus depth. Source.
Using buffer solutions to maintain constant pH.
Determining Rate from Data
Once data are collected:
Plot concentration vs. time.
Draw a tangent to the curve at the initial stage to calculate the initial rate of reaction.
Compare rates across concentrations to determine proportionality and identify the limiting reagent.
Evaluation of Experimental Design
Accuracy and Error Reduction
Improving data accuracy involves:
Using narrower concentration intervals for finer resolution.
Ensuring thorough mixing of reactants.
Calibrating equipment before use (e.g. colorimeter or gas syringe).
Repeating trials under identical conditions.
Precision: The degree to which repeated measurements under unchanged conditions show the same results.
Careful adherence to these core practical skills ensures that investigations into enzyme and substrate concentration effects produce valid, reproducible, and scientifically meaningful data.
FAQ
The initial rate is measured before substrate concentration begins to fall significantly and before product accumulation affects enzyme activity.
At this early stage:
Enzyme and substrate concentrations are known and constant.
There are minimal complications from reversible reactions or product inhibition.
This allows the rate to reflect only the direct relationship between enzyme and substrate concentrations.
Precision can be improved by:
Using digital timers and automated pipettes to reduce human error.
Employing colorimeters or spectrophotometers for objective readings rather than visual observation.
Maintaining constant temperature and pH using water baths and buffers.
Repeating each concentration multiple times and calculating a mean further enhances precision and reduces random error.
Serial dilution ensures a consistent, predictable decrease between each sample, improving accuracy and saving time.
Advantages include:
Lower chance of pipetting errors because each dilution is derived from the previous one.
Produces evenly spaced concentration steps ideal for plotting smooth rate curves.
Uses less stock solution, conserving reagents during experiments.
It’s especially useful in enzyme kinetics where fine concentration gradients reveal rate trends clearly.
At enzyme saturation, all active sites are occupied, and the rate cannot increase further.
In substrate inhibition, excessive substrate molecules distort the enzyme’s active site or block access to it, reducing activity.
While saturation maintains Vmax, substrate inhibition causes the rate to decline after reaching a peak — common at abnormally high substrate concentrations in vitro.
Systematic errors consistently skew results in one direction. In enzyme concentration investigations, they may include:
Inaccurate pipette calibration altering concentration accuracy.
Temperature fluctuations affecting enzyme activity rates.
Delayed reaction timing between samples causing inconsistent incubation periods.
Contaminated reagents or enzyme degradation leading to lower effective activity.
Using calibrated equipment, pre-warmed solutions, and consistent timing minimises these sources of bias.
Practice Questions
Question 1 (2 marks)
Describe how increasing substrate concentration affects the rate of an enzyme-catalysed reaction when enzyme concentration is kept constant.
Mark Scheme
1 mark: Rate of reaction increases as substrate concentration increases, due to more frequent enzyme–substrate collisions.
1 mark: Rate levels off (reaches Vmax) when all enzyme active sites are occupied (enzymes become saturated).
Question 2 (5 marks)
A student investigates the effect of enzyme concentration on the rate of reaction using catalase to break down hydrogen peroxide.
Describe how the student could prepare and carry out the investigation to obtain valid and reliable results.
Mark Scheme
1 mark: Prepare a range of enzyme concentrations by making serial dilutions of catalase solution.
1 mark: Keep all other variables constant (e.g. substrate concentration, temperature, pH, volume of solution).
1 mark: Measure rate of reaction by recording the volume of oxygen produced in a set time or by using a gas syringe.
1 mark: Repeat each concentration at least three times and calculate a mean rate to improve reliability.
1 mark: Plot rate of reaction against enzyme concentration to identify trends and determine when the rate plateaus due to substrate limitation.

